
- ISO 13485 INTERNAL AUDIT CHECKLIST ISO
- ISO 13485 INTERNAL AUDIT CHECKLIST PROFESSIONAL
- ISO 13485 INTERNAL AUDIT CHECKLIST DOWNLOAD
The goal, similar to FDA, is to determine compliance to ISO requirements and effective implementation of QMS best practices. In other words, ISO, too, requires organizations to conduct internal audits. The organization shall conduct internal audits at planned intervals to determine whether the quality management system: a) conforms to planned and documented arrangements, requirements of this International Standard, quality management system requirements established by the organization, and applicable regulatory requirements b) is effectively implemented and maintained.

ISO 13485:2016 Section 8.2.4 Internal audit states: Of course, all this needs to be documented and made available to the necessary stakeholders, too. Internal auditors have to be objective and initiate corrective actions as necessary. In other words, manufacturers must conduct quality audits to ensure compliance. The dates and results of quality audits and re-audits shall be documented. A report of the results of each quality audit, and reaudit(s) where taken, shall be made and such reports shall be reviewed by management having responsibility for the matters audited. Corrective action(s), including a reaudit of deficient matters, shall be taken when necessary. Quality audits shall be conducted by individuals who do not have direct responsibility for the matters being audited. Before we continue, let’s review the language each uses.Įach manufacturer shall establish procedures for quality audits and conduct such audits to assure that the quality system is in compliance with the established quality system requirements and to determine the effectiveness of the quality system.

Both FDA and ISO require manufacturers to conduct regular internal audits. Internal audits are not a “nice to have” procedure.
ISO 13485 INTERNAL AUDIT CHECKLIST DOWNLOAD
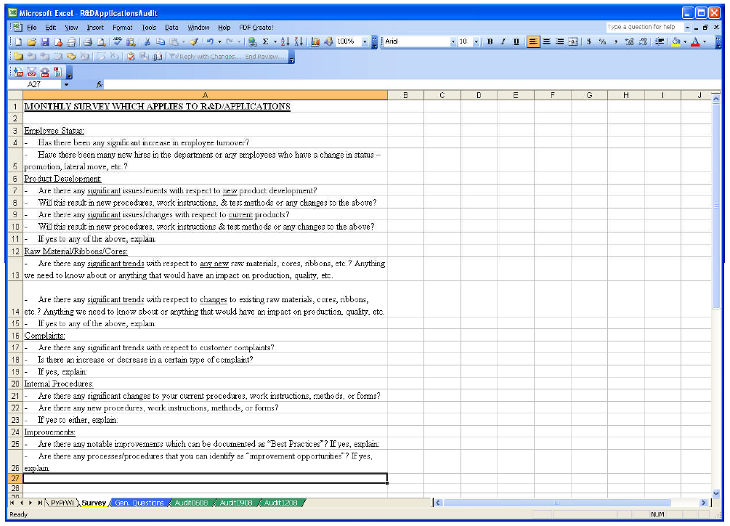
This guide will provide the ultimate internal audit checklist you can begin using today to ensure every system, process, and operation associated with your device is performing at its best.įREE RESOURCE: Click here to download a printable version of The Ultimate Internal Audit Checklist. FDA and ISO require medical device companies to conduct internal audits although the quality of these audits can vary widely. There are few times when efficiency, effectiveness, and accuracy are more important than during your internal audits. If you want your product and processes to be efficient, effective, and accurate, the checklist is the tool to use. The lesson here applies across industries, particularly for the medical device industry. Hospitals across the region saved millions of dollars and thousands of lives, all because of a simple checklist.
ISO 13485 INTERNAL AUDIT CHECKLIST PROFESSIONAL
Professional surgeon and public health researcher Atul Gawande reported, in a now-famous article for The New Yorker, that the simple act of requiring doctors to use checklists as they did their rounds helped one hospital system drop its quarterly infection rate to zero. It was nothing more than a checklist that saved $175 million and 1,500 lives.


 0 kommentar(er)
0 kommentar(er)
